Results That Matter
Team Julie delivers a remarkable outcome based on leading edge clinical science
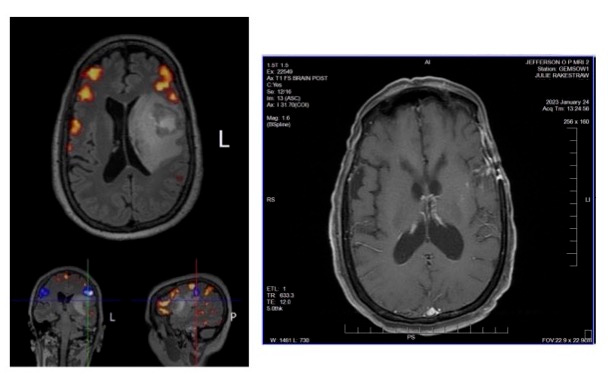
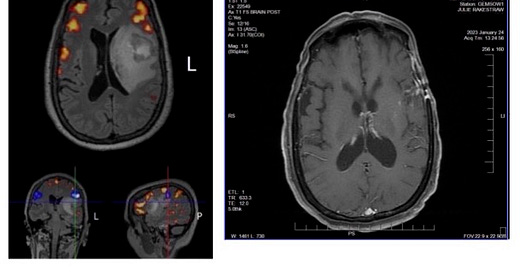
Today we share a summary of the latest scans for Julie’s GBM case (24-Jan-2023), and compare them to the fMRI scans recorded 8-Dec-2021 just prior to her maximum safe resection neurosurgery (see above photos).
Well, we must be doing something right!
From despair to hope in 14 months. Where once there was a distraught, severely aphasic patient lying on a gurney in a hospital Emergency Department as terrifying MRI scans emerged, there is now an active life to be enjoyed as a mother, friend, partner and example of what is possible with personalized treatment protocols.
We owe it all to Team Julie. There is simply no substitute for dedicated, open-minded people who are willing to collaborate and push the envelope beyond conventional thinking.
How Did We Get Here?
First, a reminder regarding the “Rules of Oncology Treatment Protocols”:
Each therapeutic protocol for an individual cancer patient is essentially an “N-of-1”, uncontrolled clinical experiment, usually involving multi-agent combination therapy. Your mileage may vary, and it is particularly challenging to rigorously attribute quantitative therapeutic effect to any single element of a combination treatment protocol. One has to commit to always following the data, and understanding that one must treat through the AEs because there are always AEs in oncology therapy. The key is to figure out how to maximize therapeutic benefit while keeping the AEs somewhat in check. Quality of life matters.
Protocol Summary
As we have previously documented on MissionGBM, Julie’s customized therapeutic regimen was designed based on deep molecular, genetic, immunological and clinical profiling. These data provided the necessary inputs upon which Team Julie could make rational, well-informed design decisions.
Tumor Treating Fields + Pembrolizumab + Fluoxetine (with Infliximab for irAE prophylaxis)
Tumor Treating Fields. Used continuously since the first day of radiation treatment on the SoC Stupp protocol (Jan-2022). Rationale: Alternating electric fields disrupt molecular scaffolds, particularly within the nuclear envelope of highly mitotic GBM cells. This electrophysical effect also has been shown to spill nuclear biomolecules into the cell cytoplasm, thus subjecting them to pro-inflammatory innate immunity pathways. The net effect is that the tumor is switched to immunologically Hot status, when makes it amenable to immunotherapy.
Pembrolizumab. Infused on a Q8W cycle. Introduced initially (without infliximab prophylaxis) following the 4-week treatment holiday after the chemo-radiation stage of the SoC Stupp protocol (Mar-2022). Rationale: Pembrolizumab is an anti-PD-1 antibody designed to inhibit immune checkpoints that would otherwise exhaust cytotoxic T-lymphocytes in the absence of immune checkpoint blockade. Julie developed multiple Grade 3+ irAEs on Cycle 1, which led to a suspension of immunotherapy until the irAEs could be fixed and a prophylaxis method was identified (Jul-2022). Infliximab was added at 5 mg/kg for each infusion chair session thereafter beginning with the first pembrolizumab rechallenge. The chair session has two sequential components: (a) infliximab goes in first over a 2 hour infusion time (Safety First!); and then (b) the bag is changed to pembrolizumab for infusion over a 30 minute period. The Q8W infusion schedule is driven by (i) the pharmacology of the treatments relative to Julie’s immunological profile; (ii) consideration of the payer policies under Julie’s insurance plan; and (iii) the convenience of a single, coordinated chair session for the Julie.
Fluoxetine. Used continuously since May-2022 on a 60mg QD PO schedule. Rationale: Pre-clinical work was published demonstrating mechanisms of action (SMPD-1 inhibitor and synergism with temozolomide) and a potential therapeutic benefit for GBM. Cleverly, the study authors also examined anonymized Episode of Treatment data from actual healthcare claims records for patients matching a GBM treatment profile to provide evidence that an Overall Survival advantage could accrue to patients taking fluoxetine. Since fluoxetine is safe and generally well-tolerated, there was no obvious downside here and an clear potential upside. The dose was chosen via allometric scaling calculations applied to the published data. Note: Independent verification of the data contained in the original paper has now been obtained, and the results have been further extended (data not yet published).
We Are Not Naïve
Cancer never sleeps. It is always working to evolve new ways of defeating the therapeutic weapons that the best medical science can throw at it.
So, we are not resting on these results. Team Julie is constantly discussing the data and ways to innovate new therapeutic agents and treatment regimens to battle evolving brain cancers. We know that there will be bumps in the road ahead, but that does not deter us.
Onward!
“N-of-1 on Behalf of All”
NOTE: MissionGBM is hosted on Substack.com servers. Substack is a business, and just like any other business, it is constantly seeking ways to grow revenue. Substack’s current business model involves receiving a modest commission from the payment stream that some authors receive for paid subscriptions to their work. However, in order for the revenue to flow, authors need to turn on the Paid Subscription feature in Substack. MissionGBM has no intention of ever charging for subscriptions. So, when you see the Pledge button auto-inserted by Substack, you can choose to ignore it (we have no way to control its auto-insertion).
Or, if you want to support brain cancer awareness, patient support/advocacy and research, please consider making a donation to the National Brain Tumor Society.