An Open Letter to Team Julie
Recognition and gratitude for a selfless group of clinicians and scientists, who make a difference in the lives of brain cancer patients every day
Dear Team Julie:
You are an amazing bunch. When life dealt our family a bad hand, you were all immediately there insisting that you would be at the table with us until we fought our way all the way back to the River card. Please allow me to explain. Pardon the Poker analogy.
In a Moment, Life Changes Forever. About a year ago, my wife Julie was diagnosed with glioblastoma multiforme (GBM), an aggressive form of brain cancer that has a bleak clinical path and an undefeated record of mortality. Patients often do not survive for a year, especially when the case presentation is as unfavorable as Julie’s. The tumor was large (5 cm) with an edematous field extending well beyond the primary tumor bed. A midline shift of >8 mm pushed a significant portion of her left cerebral hemisphere into the emergent Danger Zone of fatal brain herniation. She had rapid onset, severe aphasia – word salad, really – and struggled to communicate with me and the ER doctors. It was easy to see the fear in her eyes, even through the tears.
As the MRI data came up, I knew right away it was GBM from previous clinical research experience in the field. The scans also revealed that the inferior medial boundary of the tumor had intercalated into the lenticulostriate region proximal to the middle cerebral artery with additional impingement on the insula. Damn, that rules out a gross total resection due to unacceptable intra-operative risk of a major cerebrovascular event (stroke, hemorrhage), or abrogation of important brain executive function areas. Realistically, we would be fortunate to have Julie recover from the surgery without severely compromised speech/language and other important neurological facilities. It might be possible to perform a maximal safe resection that would remove perhaps 90% of the tumor, and that would require the skills of a world class neurosurgeon…quickly.
Family First. But first, I had to set up a conference call from the ER with our children Stephanie Rakestraw, MD and Alex Rakestraw.
When your daughter is a surgeon and you have been analyzing medical cases with her for years, there is no point in sugar-coating matters. “Show me the data, Dad! Even if it is Mom’s.”
I uploaded the video loops of the MRI and CT scans via my iPhone as the three of us talked. You could hear a pin drop. The kids quickly formulated a plan to get to airports before sunrise. Expect to drive straight from PHL to the Neuro ICU waiting area. I’ll meet you there. (Special Thanks to UAB Medicine – Surgery for working with Stephanie to cover her clinical duties in an emergency).
The Journey Begins. We had to transfer Julie overnight to a top shelf Neurosurgery department, during the height of the Delta/Omicron waves of COVID, and then stabilize her to begin the process of planning and executing the neurosurgery. The first phone calls and messages went out in the middle of the night to the initial members of Team Julie. Her case was quickly accepted by the Thomas Jefferson University Neurosurgery (NSGY) and Neuro-Oncology (NO) departments. In darkness, she was transported by ambulance to the Center City Philadelphia campus of the Jefferson Hospital for Neuroscience. Disclosure: Daughter Stephanie received her MD degree from the TJU Sidney Kimmel Medical College, so we had a leg up in terms of efficient navigation of the system.
You Can Only Look Up from the Bottom. Well after midnight, I drove home to get some food; pour a stiff drink; and lay down alone in bed for a few minutes. Sleep was impossible given the circumstances, but I had to clear my head enough to begin the process of critically thinking about the road ahead as a caregiver, husband, father and patient advocate. The amazing Joanne Smith-Farrell, PhD calls this process “The Journey”, a road traveled by all spouses/partners of a critically ill cancer patient (Disclosure: I am the President of the Joanne Smith-Farrell Fan Club of which there are many members. More on that later). Confused, our cat stood on my chest looking around for Julie.
That first night was the bottom. I had some idea of where to begin, but quickly needed the counsel of experts in the field to efficiently “Boil the Ocean” in an effort to map out Julie’s post-NSGY treatment plan. Dozens of emails and text messages were sent out worldwide asking for quick turnaround Zoom sessions and telephone calls. Team Julie began to respond immediately, often within minutes of my messages being sent (when the Team is global, someone is always awake). And, without requests from me, they amplified the call to arms by inviting their colleagues to join. Behold: The power of relationships.
One Year Later. Our Journey is now approaching a year old and still moving forward. Julie is clinically stable, active, feels good, walks 3-4 miles per day with occasional Peloton cycling sessions, and is enjoying each day despite the constant presence of her tumor (call sign “Glio”). Nearby is a recent photo of Julie celebrating her birthday with our children. The big smiles underscore commemoration of a birthday that few people believed Julie would see. Those smiles are a direct result of some incredible and selfless work performed by the amazing addressees of this Thank You note. You are all #BeyondGreat. We are forever in your debt.
What Did You Do?
If you have an interest in learning how the members of Team Julie went over-the-top to help us design and implement a therapeutic regimen customized to Julie’s clinical, molecular, genetic and immunological data, then I invite you to continue reading. It is an engaging tale told at the leading edge of research in molecular biology, genetics, immunoscience and clinical practice integrated into a personalized treatment protocol, and designed via countless hours of Zoom sessions, calls and emails spread over just about every time zone on earth.
It has not been without its setbacks – there is nothing more complex than human biology. We managed to blow up Julie’s thyroid and sigmoid colon with Grade 3+ immune-related serious adverse events (irAEs) on Cycle 1 of immunotherapy, but we recovered and kept our eyes on the horizon; testament to some breathtaking science and Julie’s courage to continue trusting the same knucklehead (#Husband) who hospitalized her early in her treatment.
We are not naïve. The Journey will undoubtedly be tortuous on the road ahead. There will be more setbacks, and additional treatment plans will need to be developed. And one day, there may be no more cards to play. However, we are aiming to be at the final table. You will know it is us: I will be the player betting all his chips on each hand, and Julie will hopefully be watching nearby.
“The Rules” Regarding Oncology Treatment Protocols
It is useful to keep in mind that each therapeutic protocol for an individual cancer patient is essentially an “N-of-1”, uncontrolled clinical experiment, usually involving multi-agent combination therapy. Your mileage may vary, and it is particularly challenging to rigorously attribute quantitative therapeutic effect to any single element of a combination treatment protocol. One has to commit to always following the data, and understanding that one must treat through the AEs because there are always AEs in oncology therapy. The key is to figure out how to maximize therapeutic benefit while keeping the AEs somewhat in check. Quality of life matters.
Cancer is a Molecular/Genetic Disease: Targeting Profile for Julie’s GBM. The first step following neurosurgery was to send the biopsy tissue taken from Julie’s tumor to multiple labs for extensive molecule and genetic profiling (FoundationOne CDx, PD-L1, TJU Molecular Typing lab, Next Gen Sequencing). A bit later, we also had to do immunological profiling to understand the molecular and cellular mechanisms behind the irAEs that hospitalized Julie in Mar-2022. Hundreds of pages of lab data had to be reviewed and integrated into the design of a customized treatment protocol. Fortunately, that is a language I speak. The essence of Julie’s tumor profile can be distilled as follows:
Tumor Mutational Burden (TMB) – Low; No druggable targets
Microsatellite Status: Stable
Unmethylated MGMT promoter – Conveys resistance to TMZ chemotherapy
wtIDH and wtTP53
PTEN Q245* alteration
>70% PD-L1 Tumor Proportion Score (TPS) – Very high for GBM; typically only seen with metastatic, end stage lung cancer, melanoma, or other non-resectable cancers
Enhanced copy number of wtEGFR/EGFRvIII and CDK4 – likely from an ecDNA origin
MDM4 and PIK3C2B amplifications
Upon seeing the molecular and genetic profiling of tissue taken from Julie’s tumor, we quickly crafted a plan to venture “off the map” following the initial 6 week chemo-radiation portion of the SoC Stupp protocol for GBM. Simply put, sticking with the post-radiation Adjuvant portion of the SoC Stupp protocol alone was not going to be a winning strategy given the molecular and genetic data.
In addition, Julie has a hyper-sensitive innate immune system. The mere shadow of a snippet of foreign nucleic acid material or microbial debris passing over her has historically led to significant immunological responses consistent with acute cytokine cascades. When this occurs, her inherently low baseline WBC counts take off causing elevated and pathological Neutrophil-to-Lymphocyte Ratios (NLR) and other sequellae. Despite never having to contend with a formal autoimmune disorder, for the purposes of immunotherapy Julie has to be viewed as equivalent to someone afflicted with a severe autoimmune condition, and treated accordingly. Silver Lining: Extensive published clinical research regarding immunotherapy of cancer patients shows that the patients displaying the most aggressive irAEs are also the ones who tend to have the best objective tumor responses.
A Brief Summary of Julie’s Personalized Treatment Protocol
DISCLAIMER: I am not a licensed physician, and I did not stay at a Holiday Inn Express last night. Nobody should interpret the following clinical summary as medical advice. We post this merely to inform, educate and potentially to serve as the basis for initiating conversations with your team of licensed healthcare providers. However, I am a PhD scientist and serial Biotech entrepreneur with decades of experience in the arenas of molecular biology, genetics, molecular medicine, immunology, translation clinical science and human clinical trials in just about every Therapeutic Area, including oncology.
Below you will find an outline of the protocol that we developed for Julie based on the specific data that we obtained from extensive molecular and genetic profiling of Julie’s GBM tumor and other organ systems. Some of the design elements in Julie’s protocol have yet to be rigorously tested in Randomized Clinical Trials (RCTs; the Gold Standard of medical science). Because each individual patient has different molecular and cellular profiles, the below should only be used to start conversations with your healthcare providers (HCPs) and care team. Protocol details, including specific dosing regimens, can be discussed during HCP peer-to-peer consultations.
T = 0 weeks: Neurosurgery (maximal safe resection); Send tissue for molecular/genetic profiling
T = 2 weeks: Data received from molecular/genetic profiling labs
T = 4 weeks: Install Optune® TTF arrays and begin treatment; Continuous use going forward
T = 4 weeks: Initiate the chemo-radiation phase of the SoC Stupp Protocol (IMRT + TMZ); Scalp-sparing IMRT conducted in the presence of installed TTF arrays
T = 10 weeks: Conclude the 6wk chemo-radiation phase of the SoC Stupp Protocol
T = 10 – 14 weeks: Treatment Holiday as per the SoC Stupp Protocol
T = 14 weeks: Begin Cycle 1 of the Adjuvant phase of the SoC Stupp Protocol; Continue for 6 monthly cycles (28 day cycles: 5 days ON, 23 days OFF)
T = 15 weeks: Initiate pembrolizumab (PZB) Cycle 1 treatment as per 2-THE-TOP protocol
T = 18 weeks: Setback – Grade 3+ irAEs (colitis and thyroiditis). Hospitalization. High dose IV corticosteroids. Suspend PZB dosing.
T = 18 – 26 weeks: Repeated attempts to wean from oral steroids. Repeated failure.
T = 20 weeks: Begin cross-taper to QD PO fluoxetine; Continuous use going forward
T = 26 weeks: First infliximab (IFX) loading infusion; Steroid wean enabled; No tumor progression via MRI
T = 26 weeks: Begin levothyroxine hormone replacement therapy; Up-titrate to steady state
T = 28 weeks: Second infliximab (IFX) loading infusion; Steroid wean completed; NLR normal; Thyroid hormones stablizing
T = 32 weeks: Third IFX infusion + First PZB rechallenge; No tumor progression via MRI
T = 38 weeks: Cycle 6 of Adjuvant TMZ. Final Cycle – Terminate TMZ dosing.
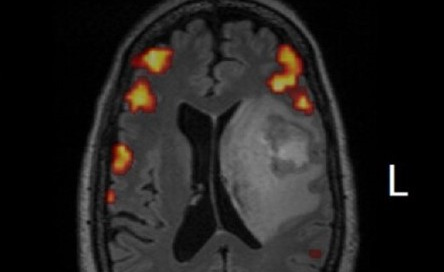
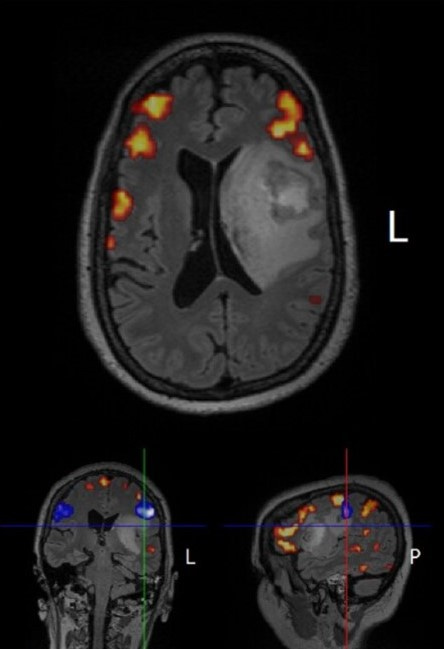
T = 39 weeks: Radiologically-confirmed improvement in tumor via MRI
T = 40 weeks and beyond: Baseline Tx regimen (pending regular clinical data reviews)
a. Optune® Tumor Treating Fields (continuous; >92% duty cycle utilization). Design Basis: FDA approved and clinically shown to extend PFS and OS via disruption of otherwise highly mitotic GBM cells.
b. Pembrolizumab (PZB) + Infliximab (IFX) – Q8W dosing cycle with sequential co-infusions in a single chair session. Design Basis: PZB is an anti-PD-1 checkpoint inhibitor which appears to work in concert with TTF to enhance PFS and OS via I/O attack on TTF-disrupted GBM cells. Reference: 2-THE-TOP Phase 2 clinical trial (NCT03405792). For Julie, IFX must be co-administered to mitigate undesirable irAEs, and permit safe and tolerable utilization of PZB.
c. Fluoxetine – Once daily administration of oral capsules. Design Basis: Fluoxetine has been reported to be a disruptor of EGFR stability and signaling in GBM cells via inhibition of the pathway enzyme SMPD1. Retrospective insurance claims database analysis suggests that GBM patients taking fluoxetine exhibit better OS than those taking other anti-depressants. Reference: Junfeng Bi, Ben Cravatt, Paul Mischel, et al, (2021) Cell Reports 37: pp 1-14 (online access).
d. Levothyroxine - Once daily administration of oral tablets. Design Basis: Required hormone replacement therapy to remediate immunotherapy-related ablation of the thyroid.
e. Levetiracetam - Twice daily administration of oral capsules. Design Basis: Seizure prophylaxis. Even though Julie has never experienced a documented seizure, most GBM patients must contend with seizures.
The Real Heroes of This Story
While more than 80 people have contributed significantly to Julie’s treatment path, the following individuals have gone over-the-top with their work and advice. We wish to publicly thank you, and to create awareness of the awesome things that you do to advance the forefront of cancer therapeutics.
Thomas Jefferson University – Sidney Kimmel Medical College (Departments of Neurosurgery, Neuro-Oncology, Radiation Oncology and Endocrinology; Philadelphia, PA).
David W. Andrews, MD is Julie’s neurosurgeon. Dr. Andrews is a respected surgeon, physician/scientist and Biotech entrepreneur – all in the service of advancing innovation to battle brain cancer. He also displays the quiet confidence that comes with decades of clinical practice, and he knows how to understand and accommodate the informational needs of the patient’s family. He quickly realized that he was free to speak candidly to us as if we were professional colleagues, involving Stephanie and me in calls regarding analysis of pre-operative data and planning of Julie’s neurosurgery. Moreover, the NSGY Fellows, Residents and nurses on Dr. Andrews’ team provided spectacular care and communication with us.
Iyad Alnahhas, MD is Julie’s local neuro-oncologist. In addition to being a skilled clinician, he has the patience of a saint, which is needed to work with your correspondent - the patient’s husband with a multi-decade background in clinical research and development. Dr. Alnahhas has always been willing to work together with us and the other external members of Team Julie to design and execute the customized treatment plan. We cannot emphasize how important this is. Too many GBM patients and their families tell us of neuro-oncologists who are uninterested in pursuing treatment strategies beyond the SoC Stupp protocol. Thank you, Dr. Alnahhas.
Voichita Bar-Ad, MD led Julie’s Radiation Oncology team. Since we initiated Tumor Treating Fields (TTF; see below) therapy with Julie on Day 1 of SoC chemo-radiation (as opposed to after the conclusion of 6 weeks of chemo-radiation), special design considerations regarding implementation of scalp-sparing IMRT in the presence of the TTF arrays on Julie’s head had to be developed. Well done.
Jeffrey L. Miller, MD is Julie’s endocrinologist. He has seen a lot of endocrine irAEs, which is exactly the experience one needs when the patient obliterates her thyroid on Cycle 1 of I/O treatment. Dr. Miller’s calm and confident presence continues to be a huge help in managing Julie through the I/O rechallenges.
David D. Tran, MD/PhD (Chief of Neuro-Oncology and Co-Director of the USC Brain Tumor Center, Keck School of Medicine at USC, Los Angeles; formerly Chief of Neuro-Oncology, McKnight Brain Institute, University of Florida College of Medicine). While I have yet to meet David in-person (hopefully that changes at SNO soon), we have spent countless hours together on Zoom, email and the phone. He is the epitome of what every physician/scientist should aspire to be – a marriage of world class clinical skills and molecular medicine research productivity that makes a difference in patients’ lives. His collaboration with Novocure to design and conduct the 2-THE-TOP clinical trial (NCT03405792) captured my attention early. His recently reported interim clinical data and J Clin Invest molecular mechanism paper are groundbreaking, suggesting that the combination of TTF and an I/O checkpoint inhibitor (pembrolizumab) can yield impressive clinical results owing to an apparent in situ vaccination of the tumor microenvironment by the action of the TTF electrical fields to induce innate immunity pathways and immunologically heat up the tumor. Julie’s immunological profiling data and clinical progress would appear to support Dr. Tran’s research. Watch this space as more clinical and non-clinical data emerges in brain cancer as well as non-brain cancer studies combining TTF with I/O therapies. Merck is partnering with Novocure to explore this promising therapeutic effect further. Many thanks to Novocure senior management (see below) for making the introduction to David, who is such a generous person that he offered to join Julie’s case from the University of Florida in early Feb-2022. David, we are forever in your debt.
Novocure (Israel, Switzerland, US). Novocure is, at all levels, one of the most innovative and patient-focused companies that I have ever encountered in my career in Biomedicine. The creativity to imagine TTF as a treatment modality for brain cancer is remarkable, and then the persistence to develop the technology through clinical trials to FDA approval is “Wow!” Every GBM patient should be aware of TTF (brand: Optune®), and working with her/his neuro-oncology team to understand how the approach might be included in a personalized treatment regimen. Yes, a caregiver must shave the patient’s head frequently in order to apply the TTF arrays, but I can tell you firsthand that it is straightforward to do once you develop your routine (I have done it more than 100 times). Recommend that you keep an eye on Novocure as it begins to announce the results of several on-going clinical trials of various TTF applications in multiple forms of cancer over the coming months. Huge Thanks to Bill Doyle (Executive Chairman) and Uri Weinberg, MD/PhD (Chief Scientific Officer) for taking the time to speak with me on several occasions, and to introduce me to Dr. David Tran. In addition, the nCompass patient support field organization is a top notch group with whom to work from the patient/caregiver perspective. Disclosure: We have an equity position in NVCR established solely as a result of the data. My remarks represent only my personal opinion based on my review of publicly available scientific, clinical and business data, and do not constitute investment advice. I receive no compensation from Novocure.
Stanford University Medical School and Innovative Medicines Accelerator (Palo Alto, CA).
Paul Mischel, MD is, to put it simply, a Mensch. His work to elucidate the molecular mechanisms of cancer biology and the importance of extra-chromosomal DNA oncogenes (ecDNA) is at the forefront of the field. Paul’s recent Cell paper regarding the promising role of the SSRI fluoxetine as a repurposed therapeutic agent to degrade the EGFR signaling pathway present in elevated copy number in almost all GBM tumors is an elegant synthesis of both state-of-the-art non-clinical data and retrospective insurance claims data analysis from actual GBM patients. While we were aware of Paul’s work in cancer, we had lots of questions. He quickly jumped on the phone with me to rigorously discuss the molecular and cellular biology data as well as the very clever retrospective patient claims data (a technique that is rarely seen in academia, but is often encouraged by WW regulatory bodies and payers). While it would have been optimal to consider adding fluoxetine to Julie’s treatment plan based on the results of a RCT, no data was available or likely to be available for years (NOTE: Let’s Go, Neuro-Oncology funding sources!; This trial needs to be initiated soon). A due diligence plan was mapped out to perform allometric dose-scaling calculations, and to investigate the safety and tolerability of fluoxetine doses as part of Julie’s treatment plan. My calculations were cross-checked with fluoxetine experts, and we began dosing Julie on fluoxetine in May while we simultaneously worked to remediate the irAE damage that was created by the first attempt at I/O in March. Just about every GBM patient needs neuropsychiatric support, and fluoxetine is a well-known anti-depressant which is broadly available worldwide. Paul and I also discussed The Journey, which further underscored Paul’s commitment to helping cancer patients.
Finally, three leading Biopharma venture capital investors, who are longtime, respected colleagues of mine, told me directly of their enthusiasm for Paul’s work. This sort of thing rarely happens, and when it does, it represents a clear signal.
Chaitan Khosla, PhD is an Institute Scholar and Professor in multiple Stanford departments. In addition, he is the Director of the Stanford Innovative Medicines Accelerator. Soon after being introduced to me via messages from mutual colleagues in the investment community, Chaitan engaged and the conversations started. The IMA is the sort of organization that deserves support and attention because it is designed and staffed to get translational results instead of just adding to the pile of basic research. I strongly urge you to check it out, and support the work of its world class researchers.
Management of irAEs. While modern I/O therapeutic agents are generally well-tolerated and represent an obvious breakthrough in cancer treatment, they are not without a companion spectrum of associated irAEs. Individual patient innate and adaptive immune systems vary significantly in terms of responses to I/O. In Julie’s case, she has a hair trigger innate immune system, which has manifested itself throughout her life. On Cycle 1 of pembrolizumab (PZB), she crashed hard 2.5 weeks after dosing with Grade 3+ irAE thyroiditis (acute, degranulation and thyroid burn out) and sigmoid colitis. A week of hospitalization with IV administration of high dose steroids and other supportive care was required to get her back on her feet and discharged. However, she was unable to complete an oral prednisone wean at home. Below 20mg QD, she experienced repeated flares of ir-Colitis and became steroid refractory, a condition which persisted for almost three months. Immune profiling revealed massive myeloid cell and neutrophil infiltrates in her colon lining with a blood Neutrophil-to Lymphocyte ratio of 12 (NLR > 3 is generally considered pathologic). OK, another problem to solve. Let’s dive into the clinical literature and start making calls. After analyzing data from dozens of papers about the role of the IL-6, JAK/STAT, TNFa and NF-kB pathways in cancer patients, some conclusions began to take shape.
Michael Dougan, MD/PhD (MGH Department of Gastroenterology & MGH Research Institute; Boston, MA). I have yet to meet Dr. Dougan in-person, which I hope to rectify as soon as I can get back to Boston. However, I have corresponded with him to let him know that his research regarding the molecular mechanisms and clinical profiles of irAEs has (1) played an enormous role in both correcting the problems that afflicted Julie on Cycle 1 of her PZB treatment; and (2) has provided the foundation for a brilliant prophylaxis strategy to enable Julie to be safely and tolerably rechallenged with PZB under a modified version of David Tran’s 2-THE-TOP clinical trial protocol. Based on Dr. Dougan’s research and clinical case reports, we designed a protocol to treat Julie with loading doses of the TNFa abrogator infliximab (IFX) to first repair the ir-Colitis damage, and then to stabilize her with background prophylaxis against further I/O-induced cytokine cascades that were attacking healthy, non-tumor tissues. It worked! Days after the first infusion of IFX, Julie’s NLR returned to a normal range; her clinical colitis symptoms dissipated; and she was able to quickly wean off oral steroids. A second loading dose of IFX per the approved label was also well-tolerated and her clinical data were strong and stable. OK, time to consider rechallenging with PZB against a prophylactic background of IFX. I reviewed Dr. Dougan’s clinical case reports and Julie’s clinical data with the Team and Julie knowing that the decision to rechallenge with PZB was solely hers to make. Remarkably, she trusted me despite the irAE misadventure back in March. We have now completed two cycles of PZB + IFX (5mpk) designed to occur as back-to-back infusions during a single extended chair session on a Q8W schedule. A third cycle is imminent. Tx regimen design considerations also included patient convenience/QoL (e.g. single infusion chair session) as well as payer-allowed claims policies regarding IFX.
Clinical Results. The therapy has been well-tolerated to date…and Julie’s latest brain MRI scans actually produced a detectable smile from her neuro-oncologist (he seldom smiles). Stay tuned as this is an evolving story. But one thing is crystal clear, Michael Dougan’s work made all of this possible. When I communicated the clinical outcome to Michael, he was simultaneously excited about the translation of his research to solve a tough clinical problem, and characteristically humble about the pivotal role that his research played in Julie’s care.
Special mention also goes to Adi Diab, MD (MD Anderson Cancer Center; Houston, TX) and Kai Wucherpfenning, MD/PhD (Dana-Farber Cancer Institute/Harvard Medical School; Boston, MA) for their outstanding work regarding the elucidation of I/O mechanisms and concomitant irAEs, which was very helpful in sorting out the immunological cascades active in Julie.
Dana-Farber Cancer Institute/Harvard Medical School (Boston, MA).
Patrick Y. Wen, MD (Director, DFCI Center for Neuro-Oncology; Professor of Neurology, HMS). Dr. Wen is a well-known leader in clinical practice and research regarding brain cancers. He is a humble, soft-spoken physician/scientist who is quick to offer help and soothing words to patients and their families. Hours after being introduce to Patrick by Bill Kaelin, he called me to discuss Julie’s case and to request a full library of MRI scans for review. The calls happened at the very beginning of my “Boil the Ocean” research to understand treatment options for Julie. We reviewed feasible options based on Julie’s clinical data, which was very helpful and a huge time saver. Dr. Wen offered to pick up Julie’s case, if we elected to move to Boston and work directly with DFCI. We have continued our conversations as Julie’s treatment marches forward and evolves, and we have added discussions regarding innovation in brain cancer medicines development. Thank You, Patrick.
William G. Kaelin, Jr., MD (DFCI, Sidney Farber Professor of Medicine, HMS). While Bill and I had overlapped previously within the context of a few Biotech newcos, I had not connected with him recently. I was familiar with Dr. Kaelin’s world class work in molecular and cellular biology but, did not realize that he, too, knew about The Journey. We found ourselves thoroughly discussing details of both the biology and clinical management of GBM as well the unique perspective that a scientist/spouse/caregiver quickly develops. Perhaps, one of the most valuable conversations that I have ever had on any topic. Bill’s advice was to speak to Patrick Wen as soon as possible, and he made the introduction. If the Nobel Prize Committee ever decides to award a prize for the combination of compassion and research excellence in Medicine and Physiology, Bill will surely be making a return trip to Stockholm.
Kirk Tanner, PhD (Chief Scientific Officer & SVP Brain Tumor Investment Fund; National Brain Tumor Society; Newton, MA). Immediately after Julie’s diagnosis, a trusted Biotech colleague and NBTS Board Director called to offer an introduction to Kirk Tanner. Kirk and I quickly formed a tight and productive bond on multiple levels. Kirk rises each day with an urgent focus on accelerating innovation and investment in the service of fighting brain cancers. While he loves to talk about the most promising technologies and clinical developments, Kirk unfortunately knows about The Journey, too. Thank You, Kirk for all the connections and introductions. I promise you that I will remain in the fight against brain cancers for years to come.
Merck & Company (Kenilworth, NJ). Merck is often viewed as the Gold Standard in pharmaceuticals R&D. MRL has been a source of breakthrough medicines over many decades, and its track record of Biopharma innovation continues today. Merck pioneered two of the drugs that have been pivotal to Julie’s treatment: Temozolomide (Temodar®) and Pembrolizumab (Keytruda®). Even more important has been Merck’s unrelenting focus on helping patients. Merck stepped up to provide expanded access to Keytruda® through its Patient Assistance Program. When we needed to ask challenging questions regarding the clinical PK and PD of PZB in order to refine Julie’s Tx protocols, Merck scientists expert in such subject matter jumped into the conversation.
While it is fashionable in some quarters to bash the Biopharma industry, I challenge anyone to sit down for coffee and conversation with a MRL scientist or clinician. You will quickly see the passion and commitment for helping patients. To quote George Merck (founder of Merck & Company): “We never try to forget that medicine is for the people. It is not for the profits.”
Disclosure: I am not a Merck employee, nor have I ever been. I do not receive any compensation from Merck, and if we own any Merck stock, it is solely via pooled investment vehicles over which we have no control. We do know a zillion Merckies around the world, and are pleased to call them our friends and colleagues. Heck, there were a couple dozen of them at our wedding 33 years ago.
We Get By With a Little Help From Our Friends
There are hundreds of people who have reached out to Julie and me in the past year to provide support and everyday assistance. We thank you all. The following three people are also my Biotech colleagues, and have distinguished themselves via their steadfast and often creative support over the past year.
Joanne Smith-Farrell, PhD (CEO – Be Biopharma; Cambridge, MA). Simply stated, Joanne is a National Treasure and fellow member of a club that neither one of us ever sought to join. Mutual friends introduced us in January shortly after Julie’s GBM diagnosis. Joanne has literally written a book about “The Journey”. She has been publicly transparent about the road traveled during her husband Matthew’s battle with GBM, and she has also shared details about the role of the spouse caregiver. Coming from a scientific background and with a track record of getting things done as a Biotech CEO and senior executive, Joanne and I can chat about things that few people have ever experienced. While our Journey is challenging, it is child’s play compared to Joanne’s. She raised three young children and worked a high responsibility Biopharma executive’s job while simultaneously acting as Matthew’s caregiver and tireless advocate for his treatment. I cannot even begin to imagine her burden. Now, you understand why I am delighted to quip that I am the President of the Joanne Smith-Farrell Fan Club of which there are many members. Thank You, Joanne: See you on Zoom again soon. The Rakestraw Family Charitable Fund is pleased to support your upcoming Kilimanjaro Climb to Fight Cancer with Luke Timmerman and several other Biopharma colleagues.
Ted Ashburn, MD/PhD (CEO – Oncorus; Cambridge, MA). Pretty much every Sunday morning Ted texts me before dawn just to check in. We met a few years ago when he contacted me out of the blue to chat about the path to becoming a first-time Biotech CEO. Dozens of meetings at our de facto office in Kendall Sq (Catalyst) forged a bond of mutual respect and invaluable discourse. Now, Ted’s drive to provide innovative treatment options to cancer patients via engineered oncolytic viruses is a major focus of our conversations. I am delighted to offer any assistance that I can.
Peter Elliott, PhD (Retired Biotech SVP of Early Development and Fearless World Traveler). Peter and I have been close friends and Biotech business associates for over 20 years, traveling the world together many times in an effort to develop new medicines for patients. We are also both reluctant sojourners on The Journey (§). For that reason, the first non-family, non-medical phone call that I made following Julie’s GBM diagnosis was to Peter. We have talked for days about what to expect and how to manage things. After our first call, Peter wrote a multi-page primer on being the spouse caregiver of a critically ill cancer patient and sent it to me. He has also buoyed Julie’s spirits daily over the past year with an endless stream of photos that he has taken during his travels to seemingly every country on earth. The first thing Julie does each morning is to check her email for Peter’s latest gifts. You give us wings, Brother.
(§) Before retiring, Peter was a well-known developer of new drugs, including oncology drugs that made a difference for countless patients (just one example: bortezomib). I remember well his passionate speech at the life celebration event for his late wife, who died from an aggressive form of hepatobiliary cancer.
“I am angry and frustrated”, he said as he addressed a room full of Biopharma executives and friends, “All of my experience and all of the science that we have at our fingertips, and yet I could ultimately do nothing to help my wife. We must try even harder.”
Those words replay in my head every day.